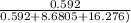Chemistry, 05.10.2019 04:20 dexterwilliams161
4. a gas containing equal parts methane, ethane and ammonia flows at a constant rate through a laboratory water- based absorption unit, which absorbs 96% of the ammonia and retains all the water. no methane or ethane is absorbed and no water evaporates. initially there was 5 l of water in the absorber, at the end of 4 hours the liquid mass is 5.25 kg. calculate the molar flowrate of the gas stream into the absorber, and the mole fraction of ammonia in the exit gas stream

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
4. a gas containing equal parts methane, ethane and ammonia flows at a constant rate through a labor...
Questions


Advanced Placement (AP), 21.09.2021 07:20


Mathematics, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20

English, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20






English, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20

Social Studies, 21.09.2021 07:20

Mathematics, 21.09.2021 07:20


Mathematics, 21.09.2021 07:20

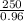 = 260.416 g
= 260.416 g
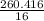 = 16.276 moles
= 16.276 moles
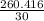 = 8.6805 moles
= 8.6805 moles
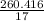 = 15.318 moles
= 15.318 moles
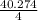 = 40.276 / 4 = 10.068 moles/h
= 40.276 / 4 = 10.068 moles/h
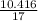 = 0.592 moles
= 0.592 moles