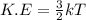Chemistry, 06.10.2019 13:30 tressasill
tori is studying a chemical reaction and its reaction rate after observing the initial rate she adds thermal energy to the reaction. which of the answer choices correctly describes how the addition of thermal energy will affect the reaction rate ?
a.) increase in energy will cause the reactance particles to move faster which will increase their temperature and lead to a faster reaction rate
b.) increase in energy will cause the reactants particles to move faster which will increase their temperature and lead to a slower reaction rate
c.) the increasing energy will cause the reactance particles to move faster which will decrease their temperature and lead to a faster reaction rate
d.) the increasing energy will cause the reactance particles to move slower which will decrease their temperature and lead to a slower reaction rate
e.) increase in energy will cause the reactants particles to move slower which will decrease their temperature and lead to a faster reaction rate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3


Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
tori is studying a chemical reaction and its reaction rate after observing the initial rate she adds...
Questions

Mathematics, 23.11.2020 22:00

Chemistry, 23.11.2020 22:00




English, 23.11.2020 22:00

History, 23.11.2020 22:00


Mathematics, 23.11.2020 22:00

Arts, 23.11.2020 22:00


History, 23.11.2020 22:00

Mathematics, 23.11.2020 22:00



Biology, 23.11.2020 22:00

Mathematics, 23.11.2020 22:00


English, 23.11.2020 22:00