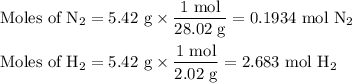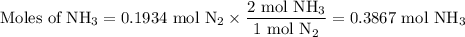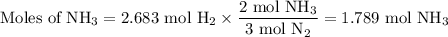N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g o...

Chemistry, 07.10.2019 00:10 ujusdied5176
N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g of hydrogen gas, which of the reactants is the limiting reactant?
molar mass of n2 = 28.02 g/mol
molar mass of h2 = 2.02 g/mol
molar mass of nh3 = 17.04 g/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Questions

Business, 26.10.2019 02:43

Physics, 26.10.2019 02:43



History, 26.10.2019 02:43




Mathematics, 26.10.2019 02:43


English, 26.10.2019 02:43

Social Studies, 26.10.2019 02:43

English, 26.10.2019 02:43

Mathematics, 26.10.2019 02:43



Physics, 26.10.2019 02:43


Mathematics, 26.10.2019 02:43

Mathematics, 26.10.2019 02:43