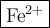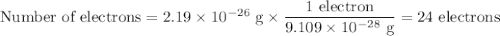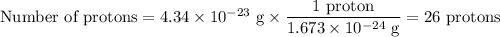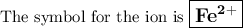Chemistry, 07.10.2019 00:10 zachstonemoreau
In an ion with an unknown charge, the total mass of all the electrons was determined to be 2.19 ✕ 10−26 g, while the total mass of its protons was 4.34 ✕ 10−23 g. what is the identity and charge of this ion? (enter your answer in the form x^q±.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
In an ion with an unknown charge, the total mass of all the electrons was determined to be 2.19 ✕ 10...
Questions

Mathematics, 08.06.2021 18:00


Mathematics, 08.06.2021 18:00

Mathematics, 08.06.2021 18:00


History, 08.06.2021 18:00

Computers and Technology, 08.06.2021 18:00

Social Studies, 08.06.2021 18:00


Mathematics, 08.06.2021 18:00

English, 08.06.2021 18:00

Mathematics, 08.06.2021 18:00

Mathematics, 08.06.2021 18:00



Health, 08.06.2021 18:00


Mathematics, 08.06.2021 18:00

Mathematics, 08.06.2021 18:00

History, 08.06.2021 18:00