
Chemistry, 07.10.2019 00:20 alisonlebron15
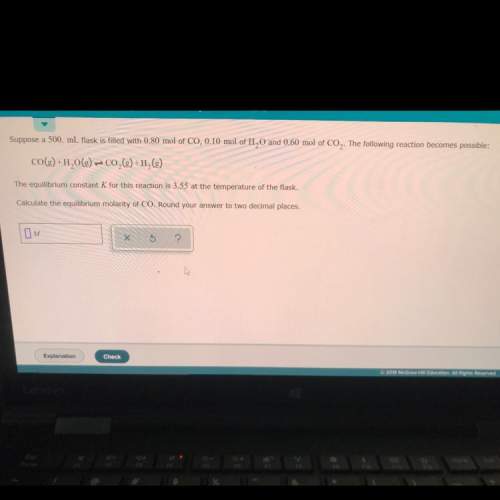
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the following reaction becomes possible:
co(g) +h2o(g) + co2(g) +h2(g)
the equilibrium constant k for this reaction is 3.55 at the temperature of the flask.
calculate the equilibrium molarity of co. round your answer to two decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the follo...
Questions



Mathematics, 26.11.2019 11:31



Mathematics, 26.11.2019 11:31


Chemistry, 26.11.2019 11:31


History, 26.11.2019 11:31


Mathematics, 26.11.2019 11:31

Physics, 26.11.2019 11:31


English, 26.11.2019 11:31

Health, 26.11.2019 11:31

History, 26.11.2019 11:31


English, 26.11.2019 11:31




