
Chemistry, 07.10.2019 01:10 sandlobster6274
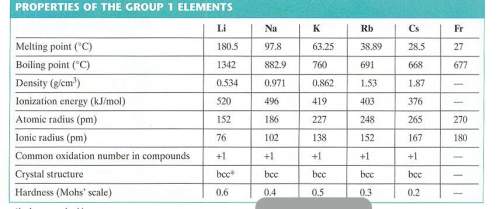
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in a row 6.00 cm long. assume that each rubidium atom touches the ones next to it.
atoms?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1
You know the right answer?
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in...
Questions

Biology, 15.06.2020 11:57


Mathematics, 15.06.2020 11:57



Mathematics, 15.06.2020 11:57


Mathematics, 15.06.2020 11:57

Biology, 15.06.2020 11:57

English, 15.06.2020 11:57

Advanced Placement (AP), 15.06.2020 11:57


Mathematics, 15.06.2020 11:57

Mathematics, 15.06.2020 11:57


Mathematics, 15.06.2020 11:57

English, 15.06.2020 12:57


Biology, 15.06.2020 12:57




