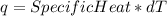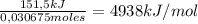Chemistry, 07.10.2019 16:10 dillondelellis2006
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/oc (including its water). the temperature inside the calorimeter was found to increase by 20.2 oc. based on this information, what is the heat of this reaction per mole of sucrose?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose...
Questions


Chemistry, 29.06.2019 05:30


Chemistry, 29.06.2019 05:30

Mathematics, 29.06.2019 05:30

History, 29.06.2019 05:30


Mathematics, 29.06.2019 05:30

English, 29.06.2019 05:30





Biology, 29.06.2019 05:30



Mathematics, 29.06.2019 05:30

Health, 29.06.2019 05:30

Mathematics, 29.06.2019 05:30