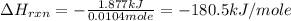Chemistry, 07.10.2019 16:20 chrismeldajbaptiste
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.22°x. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, \deltaδh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/ enter to 1 decimal place.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648...
Questions



Mathematics, 10.04.2020 01:26

Mathematics, 10.04.2020 01:26

Law, 10.04.2020 01:26


Mathematics, 10.04.2020 01:26

Mathematics, 10.04.2020 01:26




English, 10.04.2020 01:26

Mathematics, 10.04.2020 01:26


Mathematics, 10.04.2020 01:26

History, 10.04.2020 01:26


Mathematics, 10.04.2020 01:26


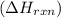 is, -180.5 kJ/mole
is, -180.5 kJ/mole
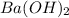
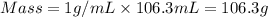
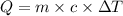
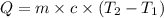
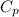 = specific heat capacity of water =
= specific heat capacity of water = 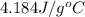
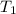 = initial temperature =
= initial temperature = 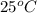
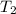 = final temperature =
= final temperature = 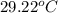
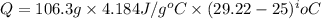
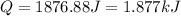 (1 kJ = 1000 J)
(1 kJ = 1000 J)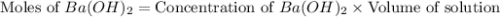
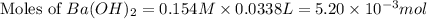
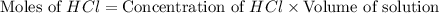
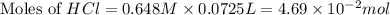
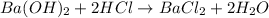
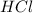
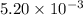 moles of
moles of 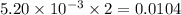 moles of
moles of 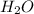
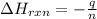
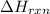 = enthalpy of reaction = ?
= enthalpy of reaction = ?