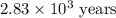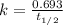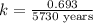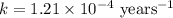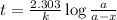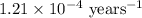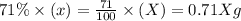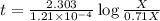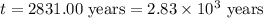Chemistry, 07.10.2019 17:30 animationfusion
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that has a half-life of 5730 years. if a sample containing carbon-14 now has 71% of its original concentration of carbon-14, how much time has passed in years? 4.09 ~ 103 years 5.73 x 103 years 2.38 x 103 years 2.83 x 103 years 3.52 * 104 years

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

You know the right answer?
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that h...
Questions



Mathematics, 16.10.2019 06:20

Mathematics, 16.10.2019 06:20

Biology, 16.10.2019 06:20








Mathematics, 16.10.2019 06:20



English, 16.10.2019 06:20


Mathematics, 16.10.2019 06:20


Computers and Technology, 16.10.2019 06:20