
Chemistry, 07.10.2019 18:30 oliviaclerk5
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable binary ionic compound. is he correct? why or why not?
a hector is incorrect. the two ions have opposite charges, so they will bond together in a binary ionic compound.
b hector is incorrect. the two ions have more than eighteen protons, so they will bond together in a binary ionic compound.
c hector is correct. the sum of the charges of the two ions must be zero in order for them to form a stable ionic compound.
d hector is correct. the sum of the protons of the two ions must be divisible by eight in order for them to form a stable ionic compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable...
Questions







Biology, 26.09.2019 08:20


English, 26.09.2019 08:20

Mathematics, 26.09.2019 08:20

Mathematics, 26.09.2019 08:20

Mathematics, 26.09.2019 08:20



Mathematics, 26.09.2019 08:20

Health, 26.09.2019 08:20


Biology, 26.09.2019 08:20

History, 26.09.2019 08:20

![[Na]=1s^22s^22p^63s^1](/tpl/images/0297/6892/4ee3b.png)
![[O]=1s^22s^22p^4](/tpl/images/0297/6892/336ff.png)
![[O^{2-}]=1s^22s^22p^6](/tpl/images/0297/6892/c797e.png)
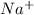 cation and oxide
cation and oxide 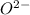 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral 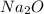 .
.

