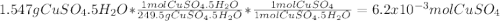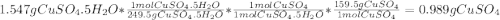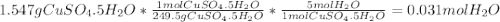Chemistry, 07.10.2019 19:10 jeffro198004
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the water. the white crystals of that are left behind have a mass of g. how many moles of were in the original sample? show that the relative molar amounts of and agree with the formula of the hydrate.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

You know the right answer?
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the wa...
Questions

Mathematics, 19.05.2020 02:03


History, 19.05.2020 02:03


Mathematics, 19.05.2020 02:03

Mathematics, 19.05.2020 02:03


Biology, 19.05.2020 02:03

Biology, 19.05.2020 02:03







Mathematics, 19.05.2020 02:03


Mathematics, 19.05.2020 02:03

Mathematics, 19.05.2020 02:03

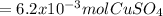
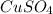 :
: