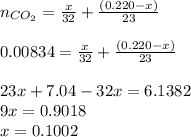An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5oh ). a 0.220-g m g sample of the liquid is burned in an excess of o2(g) m o_2(g) and yields 0.367g g co2(g) m co_2(g) (carbon dioxide). set up two algebraic equations, one expressing the mass of carbon dioxide produced in terms of each reagent and the other expressing the mass of sample burned in terms of each reagent.
what is the mass of methyl alcohol (ch3oh m ch_3oh) in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1


Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5...
Questions


Chemistry, 25.02.2021 03:10

Mathematics, 25.02.2021 03:10

Chemistry, 25.02.2021 03:10


Advanced Placement (AP), 25.02.2021 03:10



Mathematics, 25.02.2021 03:10

English, 25.02.2021 03:10

Mathematics, 25.02.2021 03:10

Chemistry, 25.02.2021 03:10

Mathematics, 25.02.2021 03:10


Mathematics, 25.02.2021 03:10


French, 25.02.2021 03:10



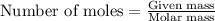 .....(1)
.....(1)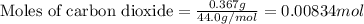
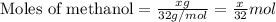
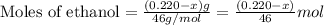
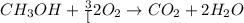
![\frac{x}{32} moles of methanol will produce = [tex]\frac{1}{1}\times \frac{x}{32}=\frac{x}{32}](/tpl/images/0297/8500/66d68.png) moles of carbon dioxide
moles of carbon dioxide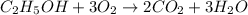
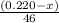 moles of ethanol will produce =
moles of ethanol will produce = 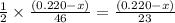 moles of carbon dioxide
moles of carbon dioxide