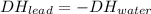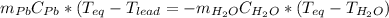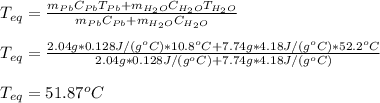Chemistry, 07.10.2019 19:30 amayarayne5
A2.04 g lead weight, initially at 10.8 oc, is submerged in 7.74 g of water at 52.2 oc in an insulated container. clead = 0.128 j/goc; cwater = 4.18 j/goc. what is the final temperature of both the weight and the water at thermal equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

You know the right answer?
A2.04 g lead weight, initially at 10.8 oc, is submerged in 7.74 g of water at 52.2 oc in an insulate...
Questions

Chemistry, 16.07.2019 16:00

History, 16.07.2019 16:00



Mathematics, 16.07.2019 16:00


Geography, 16.07.2019 16:00







Mathematics, 16.07.2019 16:00

History, 16.07.2019 16:00

Mathematics, 16.07.2019 16:00

Mathematics, 16.07.2019 16:00



Mathematics, 16.07.2019 16:00