
Chemistry, 07.10.2019 22:00 alayciaruffin076
To use combustion analysis data to determine an empirical formula a molecular formula expresses the number of each kind of atom in a molecule. for example, the molecular formula for propene, c3h6, indicates three carbon atoms and six hydrogen atoms per molecule. this also means that one mole of propene contains three moles of carbon and six moles of hydrogen. an empirical formula expresses the mole ratio of the elements. the empirical formula for propene is ch2, indicating twice as much hydrogen as carbon. when analyzing unknown compounds in a lab, it is often possible to identify the mole ratios, and thus the empirical formula, but not the molecular formula. notice that the molecular mass of propene, 3(12)+6(1)=42amu, is a multiple of the empirical formula mass, 1(12)+2(1)=14amu . an unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.00 g of this compound produced 4.40 g of carbon dioxide and 1.80 g of water. how many moles of carbon, c, were in the original sample? how many moles of hydrogen, h, were in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
To use combustion analysis data to determine an empirical formula a molecular formula expresses the...
Questions


Mathematics, 19.07.2020 01:01


History, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01


Biology, 19.07.2020 01:01


Mathematics, 19.07.2020 01:01


Computers and Technology, 19.07.2020 01:01






Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

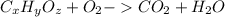
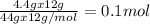 of Carbon
of Carbon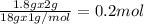 of Hydrogen
of Hydrogen

