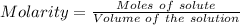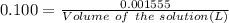According to the balanced chemical equation
5 h2c2o4(aq) + 2 mno4-(aq) + 6 h+(aq) → 10 co2(g) + 2 mn2+(aq) + 8 h2o(l)
0.3500 grams of oxalic acid, h2c2o4 will react with ml of 0.100 m potassium permanganate, kmno4 solution.
a) 15.5 ml
b) 38.9 ml
c) 77.7 ml
d) 97.2 ml

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
According to the balanced chemical equation
5 h2c2o4(aq) + 2 mno4-(aq) + 6 h+(aq) → 10 c...
5 h2c2o4(aq) + 2 mno4-(aq) + 6 h+(aq) → 10 c...
Questions


Mathematics, 11.09.2019 04:30

Social Studies, 11.09.2019 04:30






English, 11.09.2019 04:30


Computers and Technology, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30




Mathematics, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

Chemistry, 11.09.2019 04:30

Computers and Technology, 11.09.2019 04:30

Mathematics, 11.09.2019 04:30

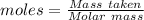
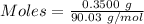
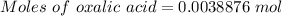
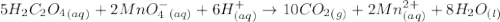
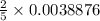 moles of potassium permanganate
moles of potassium permanganate