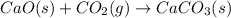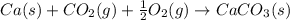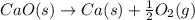Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) how should you manipulate these equations so that they produce the equation below when added? check all that apply. cao(s) + co2(g) → caco3(s) reverse the direction of equation (2) multiply equation (1) by 3 multiply equation (2) by 1/2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) h...
Questions


Mathematics, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00




Mathematics, 28.01.2021 01:00



Mathematics, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

English, 28.01.2021 01:00




Mathematics, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00