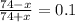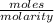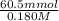Chemistry, 07.10.2019 23:00 crazymadhatter0
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or base is added. suppose that you have 185 ml of a buffer that is 0.400 m in both acetic acid (ch3cooh) and its conjugate base (ch3coo−) . calculate the maximum volume of 0.180 m hcl that can be added to the buffer before its buffering capacity is lost.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or ba...
Questions




History, 26.07.2019 01:30

Biology, 26.07.2019 01:30

Mathematics, 26.07.2019 01:30

Mathematics, 26.07.2019 01:30


English, 26.07.2019 01:30



Spanish, 26.07.2019 01:30





English, 26.07.2019 01:30

Biology, 26.07.2019 01:30

Biology, 26.07.2019 01:30

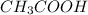 and
and 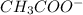 .
.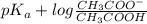
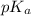 value of acetic acid is 4.76.
value of acetic acid is 4.76. 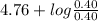
![[CH_{3}COOH]](/tpl/images/0298/3853/d21d8.png) the log term gives a negative value. This means that new pH will be less than 4.76.
the log term gives a negative value. This means that new pH will be less than 4.76.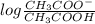
 = 0.1 ....... (1)
= 0.1 ....... (1)