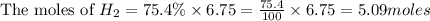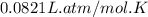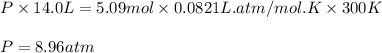Chemistry, 07.10.2019 23:00 brandytyler317fries
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what is the pressure of h2 if the hydrogen gas collected occupies 14.0 l at 300.k and was produced upon reaction of 4.50 moles of al and excess hcl in a process that has a 75.4 percent yield?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what i...
Questions

English, 12.08.2021 01:00


Mathematics, 12.08.2021 01:00


English, 12.08.2021 01:00


Mathematics, 12.08.2021 01:00

Biology, 12.08.2021 01:00

Computers and Technology, 12.08.2021 01:00



Chemistry, 12.08.2021 01:00


Social Studies, 12.08.2021 01:00

Chemistry, 12.08.2021 01:00



Mathematics, 12.08.2021 01:00


Arts, 12.08.2021 01:00

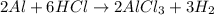
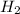 gas
gas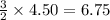 moles of
moles of