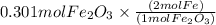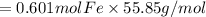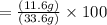Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1
You know the right answer?
Combining 0.301 mol fe2o3
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
with excess carbon produced 11.6 g fe.
fe2o3+3c⟶2fe+3co<...
Questions

Mathematics, 30.01.2020 18:55

Chemistry, 30.01.2020 18:55


History, 30.01.2020 18:55

Mathematics, 30.01.2020 18:56

Mathematics, 30.01.2020 18:56

Social Studies, 30.01.2020 18:56


English, 30.01.2020 18:56


Mathematics, 30.01.2020 18:56

Mathematics, 30.01.2020 18:56

History, 30.01.2020 18:56



Biology, 30.01.2020 18:56


Mathematics, 30.01.2020 18:56


Biology, 30.01.2020 18:56

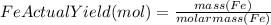
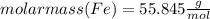
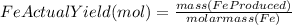
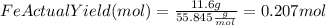
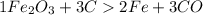
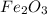 to moles Fe and moles Fe to mass Fe
to moles Fe and moles Fe to mass Fe 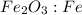 is 1 : 2
is 1 : 2