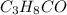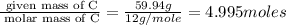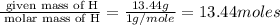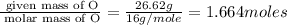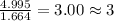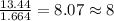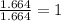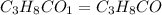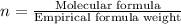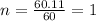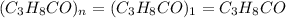Chemistry, 07.10.2019 22:30 MichaelBoolin87241
Acompound is found to contain 59.94 % carbon , 13.44 % hydrogen , and 26.62 % oxygen by mass.
(1) the empirical formula for this compound is .
(2) the molecular weight for this compound is 60.11 amu. the molecular formula for this compound is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
Acompound is found to contain 59.94 % carbon , 13.44 % hydrogen , and 26.62 % oxygen by mass.
...
...
Questions

Mathematics, 16.12.2020 20:50



History, 16.12.2020 20:50



History, 16.12.2020 20:50

English, 16.12.2020 20:50


Mathematics, 16.12.2020 20:50





Mathematics, 16.12.2020 20:50

Physics, 16.12.2020 20:50

History, 16.12.2020 20:50


Mathematics, 16.12.2020 20:50

German, 16.12.2020 20:50