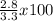Chemistry, 08.10.2019 01:00 algahimnada
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: cao(s) h2o(l) → ca(oh)2(s) in a particular experiment, a 2.50-g sample of cao is reacted with excess water and 2.80 g of ca(oh)2 is recovered. what is the percent yield in this experiment

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: cao(s) h2o(...
Questions


Mathematics, 17.01.2020 16:31


Mathematics, 17.01.2020 16:31






Mathematics, 17.01.2020 16:31

English, 17.01.2020 16:31

English, 17.01.2020 16:31



English, 17.01.2020 16:31

History, 17.01.2020 16:31

Mathematics, 17.01.2020 16:31

Mathematics, 17.01.2020 16:31

Mathematics, 17.01.2020 16:31