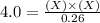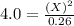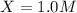Chemistry, 08.10.2019 01:00 hamadehassan
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l container and allowed to come to equilibrium, and the equilibrium concentration of pcl5(g) is 0.26 m, what is the equilibrium concentration of pcl3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l...
Questions

Mathematics, 02.11.2020 22:40


Mathematics, 02.11.2020 22:40




Mathematics, 02.11.2020 22:40





Mathematics, 02.11.2020 22:40


Mathematics, 02.11.2020 22:40

Social Studies, 02.11.2020 22:40


Mathematics, 02.11.2020 22:40


History, 02.11.2020 22:40

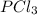 is, 1.0 M
is, 1.0 M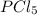 = 0.26 M
= 0.26 M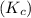 = 4.0
= 4.0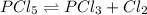
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0298/6790/73fe0.png)
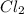 are equal.
are equal.