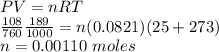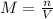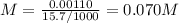The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved in about 60 ml of water and titrated with an naoh solution; 15.7 ml of the naoh solution were required to neutralize the hcl. calculate the molarity of the naoh solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved...
Questions


History, 12.05.2021 05:00





Mathematics, 12.05.2021 05:00

Mathematics, 12.05.2021 05:00

History, 12.05.2021 05:00



Mathematics, 12.05.2021 05:00




Mathematics, 12.05.2021 05:00

Mathematics, 12.05.2021 05:00

English, 12.05.2021 05:00


Arts, 12.05.2021 05:00