
Chemistry, 08.10.2019 02:30 iwantcandy2002
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lime slurries of calcium hydroxide to remove the so2 from the flue gases. write the balanced equation for the reaction between solid calcium hydroxide and so2. include the states of all reactants and products in your equation. now, calculate the δs o at 298 k [s o of caso3(s) = 101.4 j/mol k].

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lim...
Questions

History, 11.01.2020 14:31




Spanish, 11.01.2020 14:31

Mathematics, 11.01.2020 14:31

English, 11.01.2020 14:31

Mathematics, 11.01.2020 14:31


English, 11.01.2020 14:31

Chemistry, 11.01.2020 14:31

Mathematics, 11.01.2020 14:31

Mathematics, 11.01.2020 14:31

Physics, 11.01.2020 14:31

History, 11.01.2020 14:31

Biology, 11.01.2020 14:31

Mathematics, 11.01.2020 14:31

Biology, 11.01.2020 14:31


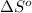 for the reaction is -160.6 J/K
for the reaction is -160.6 J/K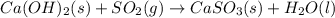
![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0298/9458/e71e2.png)
![\Delta S^o_{rxn}=[(1\times \Delta S^o_{CaSO_3(s)})+(1\times \Delta S^o_{H_2O(l)})]-[(1\times \Delta S^o_{Ca(OH)_2(s)})+(1\times \Delta S^o_{SO_2(g)})]](/tpl/images/0298/9458/6c149.png)
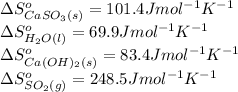
![\Delta S^o_{rxn}=[(1\times (101.4))+(1\times (69.9))]-[(1\times (83.4))+(1\times (248.5))]\\\\\Delta S^o_{rxn}=-160.6J/K](/tpl/images/0298/9458/59a05.png)


