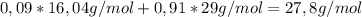Chemistry, 08.10.2019 03:10 robert7248
Amixture of methane and air is capable of being ignited only if the mole percent of methane is between 5% and 15%. a mixture containing 9.00 mole% methane in air flowing at a rate of 700.0 kg/h is to be diluted with pure air to reduce the methane concentration to the lower flammability limit. calculate the required flow rate of air in mol/h and the percent by mass of oxygen in the product gas. (note: air may be taken to consist of 21.0 mole% o2 and 79.0 mole% n2 and hence to have an average molecular weight of 29.0).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Amixture of methane and air is capable of being ignited only if the mole percent of methane is betwe...
Questions

Mathematics, 24.06.2019 10:00



English, 24.06.2019 10:00



Health, 24.06.2019 10:00

Mathematics, 24.06.2019 10:00

Mathematics, 24.06.2019 10:00



Biology, 24.06.2019 10:00

English, 24.06.2019 10:00