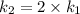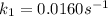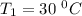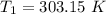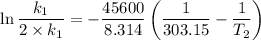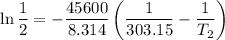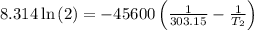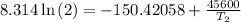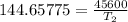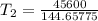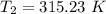Chemistry, 08.10.2019 03:20 jonlandis6
The arrhenius equation shows the relationship between the rate constant k and the temperature t in kelvins and is typically written as k=ae−ea/rt where r is the gas constant (8.314 j/mol⋅k), a is a constant called the frequency factor, and ea is the activation energy for the reaction. however, a more practical form of this equation is lnk2k1=ear(1t1−1t2) which is mathematically equivalent to lnk1k2=ear(1t2−1t1) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (t1 and t2). part a the activation energy of a certain reaction is 45.6 kj/mol . at 30 ∘c , the rate constant is 0.0160s−1 . at what temperature in degrees celsius would this reaction go twice as fast

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

You know the right answer?
The arrhenius equation shows the relationship between the rate constant k and the temperature t in k...
Questions

History, 10.03.2021 20:20


English, 10.03.2021 20:20

Mathematics, 10.03.2021 20:20

Mathematics, 10.03.2021 20:20

Mathematics, 10.03.2021 20:20


Social Studies, 10.03.2021 20:20

History, 10.03.2021 20:20



Mathematics, 10.03.2021 20:20

Social Studies, 10.03.2021 20:20



Mathematics, 10.03.2021 20:20



Mathematics, 10.03.2021 20:20

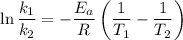
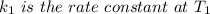
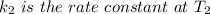
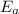 is the activation energy
is the activation energy