
Chemistry, 08.10.2019 03:30 duncanje5783
Be sure to answer all parts. determine the overall orders of the reactions to which the following rate laws apply: (a) rate = k[no2]2 (b) rate = k zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order (c) rate = k[h2][br2]1/2 (d) rate = k[no2]2[o2] zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2


Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Be sure to answer all parts. determine the overall orders of the reactions to which the following ra...
Questions



Mathematics, 22.10.2020 08:01

History, 22.10.2020 08:01

Advanced Placement (AP), 22.10.2020 08:01



History, 22.10.2020 08:01



History, 22.10.2020 08:01

Geography, 22.10.2020 08:01

Computers and Technology, 22.10.2020 08:01

Biology, 22.10.2020 08:01

Physics, 22.10.2020 08:01

Advanced Placement (AP), 22.10.2020 08:01




Mathematics, 22.10.2020 08:01

![r=k[NO_2]^2](/tpl/images/0299/0794/8772b.png)
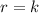
![r=k[H_2]*[Br_2]^{1/2}](/tpl/images/0299/0794/34ab2.png)
![r=k[NO_2]^2[O_2]](/tpl/images/0299/0794/ccd53.png)


