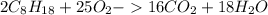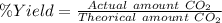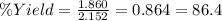Chemistry, 08.10.2019 05:00 justin5647
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane (c_8 8 h_{18} 18 ) react with excess oxygen to produce 81.75 g of co_2 2 (g) 2c_8 8 h_{18} 18 (i) + 25o_2 2 (g) → 16co_2 2 (g) + 18h_2 2 o(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2

You know the right answer?
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane...
Questions


Chemistry, 07.12.2020 14:00





Mathematics, 07.12.2020 14:00

Biology, 07.12.2020 14:00


Arts, 07.12.2020 14:00

Mathematics, 07.12.2020 14:00

Mathematics, 07.12.2020 14:00




Health, 07.12.2020 14:00

Mathematics, 07.12.2020 14:00

Social Studies, 07.12.2020 14:00