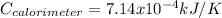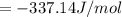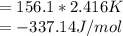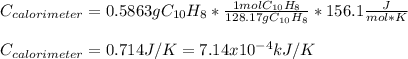When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter constant, a temperature rise of 2.862 k was measured near 298k. under similar conditions, a temperature rise of 2.416 k was measured when a 0.5863 g naphthalene sample was burned. determine the calorimeter constant (in units of kj/k) and the standard enthalpy of combustion for naphthalene at 298k. if we make the assumption that ∆h ≈∆u, is the ∆chº value obtained from this experim

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter co...
Questions


Mathematics, 24.04.2020 18:06


Mathematics, 24.04.2020 18:06




History, 24.04.2020 18:06

Mathematics, 24.04.2020 18:06

Mathematics, 24.04.2020 18:06


Biology, 24.04.2020 18:06

History, 24.04.2020 18:06

Computers and Technology, 24.04.2020 18:06

Mathematics, 24.04.2020 18:06

History, 24.04.2020 18:06

Mathematics, 24.04.2020 18:06