
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to remove h2s is to treat the water with chlorine, in which case the following reaction occurs: h2s(aq)+cl2(aq)→s(s)+2h+(aq)+2cl−(a q) the rate of this reaction is first order in each reactant. the rate constant for the disappearance of h2s at 28 ∘c is 3.5×10−2 m−1s−1.if at a given time the concentration of h2s is 2.0×10-4 m and that of cl2 is 2.8×10-2 m , what is the rate of formation of cl?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to r...
Questions

Biology, 15.01.2020 04:31

Mathematics, 15.01.2020 04:31

History, 15.01.2020 04:31

Mathematics, 15.01.2020 04:31



English, 15.01.2020 04:31

Mathematics, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31


Computers and Technology, 15.01.2020 04:31


History, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31

Mathematics, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31

Chemistry, 15.01.2020 04:31

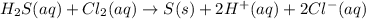
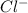 will be twice the rate of disappearance of
will be twice the rate of disappearance of 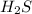 .
.![k[H_{2}S][Cl_{2}]](/tpl/images/0299/3822/2cbd6.png)
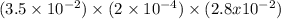
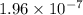 M/s
M/s
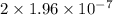
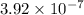 M/s
M/s

