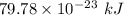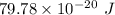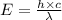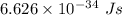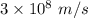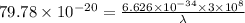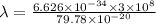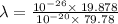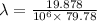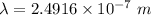Chemistry, 08.10.2019 17:30 newtonthenewt
The work function of an element is the energy required to remove an electron from the surface of the solid. the work function for rhodium is 480.5 kj/mol (that is, it takes 480.5 kj of energy to remove 1 mole of electrons from 1 mole of rh atoms on the surface of rh metal). what is the maximum wavelength of light that can remove an electron from an atom in rhodium metal?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 13:30
Which factors would have influenced earth’s climate during the time of pangea? check all that apply. land covered by glaciers one large landmass one large ocean tectonic activity multiple small seas
Answers: 2

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1
You know the right answer?
The work function of an element is the energy required to remove an electron from the surface of the...
Questions


Mathematics, 22.04.2020 23:04




English, 22.04.2020 23:04

Mathematics, 22.04.2020 23:04

Mathematics, 22.04.2020 23:04

Biology, 22.04.2020 23:04


Mathematics, 22.04.2020 23:04




Mathematics, 22.04.2020 23:04

Social Studies, 22.04.2020 23:04

Mathematics, 22.04.2020 23:04



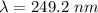
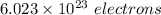
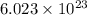 electrons can be removed by applying of 480.5 kJ of energy.
electrons can be removed by applying of 480.5 kJ of energy.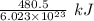 of energy.
of energy.