
Chemistry, 08.10.2019 18:20 memester74
The equilibrium constant k for a certain reversible reaction is 1.32 × 10−4. which of the following is true for the reaction? the reverse reaction is more favorable than the forward reaction. the forward reaction takes place at a much faster rate than the reverse reaction. the equilibrium reaction mixture will have a very low concentration of reactants. the equilibrium reaction mixture will have a very high concentration of products.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
The equilibrium constant k for a certain reversible reaction is 1.32 × 10−4. which of the following...
Questions

Social Studies, 09.11.2020 19:40


Chemistry, 09.11.2020 19:40


Mathematics, 09.11.2020 19:40

Health, 09.11.2020 19:40


Mathematics, 09.11.2020 19:40


Mathematics, 09.11.2020 19:40

World Languages, 09.11.2020 19:40

Physics, 09.11.2020 19:40




Biology, 09.11.2020 19:40

Geography, 09.11.2020 19:40



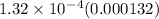 . A high value of equilibrium constant indicates that the forward reaction is more favourable.
. A high value of equilibrium constant indicates that the forward reaction is more favourable. 

