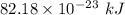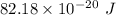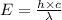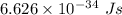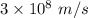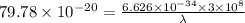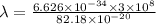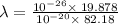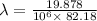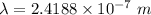Chemistry, 08.10.2019 19:00 autumnskye1
It takes 495.0 kj of energy to remove 1 mole of electron from an atom on the surface of sodium metal. how much energy does it take to remove a single electron from an atom on the surface of solid sodium? energy = j. what is the maximum wavelength of light capable of doing this?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
It takes 495.0 kj of energy to remove 1 mole of electron from an atom on the surface of sodium metal...
Questions


Mathematics, 08.11.2020 19:40


Mathematics, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40

Arts, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40



Physics, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40






Biology, 08.11.2020 19:40

Mathematics, 08.11.2020 19:40

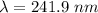
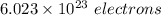
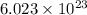 electrons can be removed by applying of 495.0 kJ of energy.
electrons can be removed by applying of 495.0 kJ of energy.
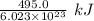 of energy.
of energy.