
Chemistry, 08.10.2019 21:30 NaVaThEBeAsT
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) how many moles of fe can be made from 1.25 moles of fe2o3? (b) how many moles of h2 are needed to make 3.75 moles of fe? (c) if the reaction yields 12.50 moles of h2o, what mass of fe2o3 was used

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) ho...
Questions



Mathematics, 24.04.2020 21:04












Health, 24.04.2020 21:04



English, 24.04.2020 21:04

History, 24.04.2020 21:04

History, 24.04.2020 21:04

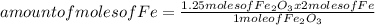
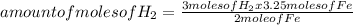
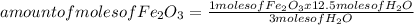
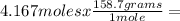 665.4699 grams
665.4699 grams

