
Chemistry, 09.10.2019 02:00 tylerineedhelp
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a second order reaction, the half-life is 726 seconds. starting with a concentration of 0.600 m, how long will it take for the concentration to decrease to 0.150 m? c. the half-life for a first order reaction is 2768 years. starting with a concentration of 0.345 m, what will the concentration be after 11,072 years? d. the half-life for first order reaction a is 75 minutes. the half-life for a different first order reaction b is 322 minutes. which reaction has the faster rate? (a or b) which reaction has the larger rate constant, k? (a or b)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a secon...
Questions





Social Studies, 10.07.2019 01:00

Social Studies, 10.07.2019 01:00






Social Studies, 10.07.2019 01:00






Mathematics, 10.07.2019 01:00


Chemistry, 10.07.2019 01:00

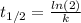 (1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹
(1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹

