
Chemistry, 09.10.2019 03:30 fancycar14
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed to oxygen. the reaction is 2b5h9(l) + 12o2(g) ⟶ 5b2o3(s) + 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formation of b5h9 is 73.2 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed...
Questions





Mathematics, 05.11.2019 02:31


Chemistry, 05.11.2019 02:31












Advanced Placement (AP), 05.11.2019 02:31

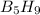 is -71.92 kJ
is -71.92 kJ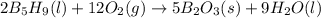
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0302/4270/72c39.png)
![\Delta H^o_{rxn}=[(5\times \Delta H^o_f_{(B_2O_3(s))})+(9\times \Delta H^o_f_{(H_2O(l))})]-[(2\times \Delta H^o_f_{(B_5H_9(l))})+(12\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0302/4270/e310e.png)
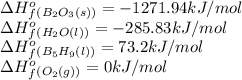
![\Delta H^o_{rxn}=[(5\times (1271.94))+(9\times (-285.83))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H^o_{rxn}=-9078.57kJ](/tpl/images/0302/4270/015ae.png)
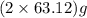 of
of 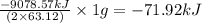 of energy.
of energy.

