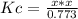Chemistry, 09.10.2019 05:00 katiedurden02
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it decomposes by the reaction: cocl2 (g) ⇋ co (g) + cl2 (g) kc = 7.5 x 10-5 (at 362°c) calculate the concentration of co when 7.73 mol of phosgene decomposes and reaches equilibrium in a 10.0 liter flask. multiply your answer by 103 and enter that number to 2 decimal places. hint: look at sample problem 17.9 in the 8th ed of silberberg. write a kc expression. calculate the initial concentration of phosgene. do an ice chart. find the concentration of co.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 12:50
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1
You know the right answer?
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it deco...
Questions

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30



Social Studies, 18.03.2021 02:30




Mathematics, 18.03.2021 02:30


History, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30




Mathematics, 18.03.2021 02:30


![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0302/6261/d3f86.png)