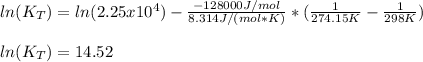Chemistry, 09.10.2019 16:30 parkerfreeze
Enter your answer in the provided box. the formation of methanol is important to the processing of new fuels. at 298.0 k, kp = 2.25 × 104 for the reaction co(g) + 2 h2(g) ⇌ ch3oh(l) if δh o rxn = −128 kj/ mol ch3oh, calculate kp at 1°c. × 10 -6 (enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Enter your answer in the provided box. the formation of methanol is important to the processing of n...
Questions

Mathematics, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31






Social Studies, 29.11.2019 14:31

Geography, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31


Mathematics, 29.11.2019 14:31


Chemistry, 29.11.2019 14:31

Geography, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31


Mathematics, 29.11.2019 14:31

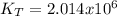
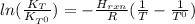
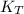 it means the equilibrium constant at 1 °C, we get:
it means the equilibrium constant at 1 °C, we get: