
Chemistry, 09.10.2019 19:10 ohartshorn1599
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfide, na2s. will all the lead be removed from 11.2 ml of 7.10×10-3 m pb(no3)2 upon addition of 12.4 ml of 0.0117 m na2s? if all the lead is removed, how many moles of lead is this? if not, how many moles of pb remain?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfi...
Questions






History, 22.09.2021 19:00

Physics, 22.09.2021 19:00

Mathematics, 22.09.2021 19:00








Mathematics, 22.09.2021 19:00

Social Studies, 22.09.2021 19:00


Mathematics, 22.09.2021 19:00

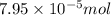
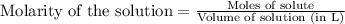
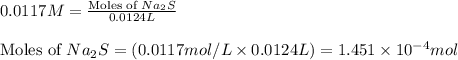
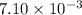 M
M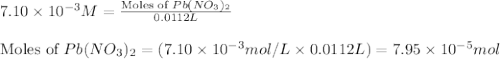
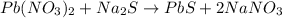
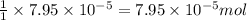 of sodium sulfide.
of sodium sulfide.

