
Consider the overall reaction: 2 x2y + z2 ⇌ 2 x2yz which has an experimentally determined rate law of: rate= k[x2y][z2]. which of the following are possible mechanisms for the reaction? group of answer choices step 1: 2 x2y + z2 → 2x2yz (slow) step 1: x2y + z2 ⇌ x2yz + z (fast) step 2: x2y + z → x2yz (slow) step 1: x2y + z2→ x2yz2 (slow) step 2: x2yz2 + x2y → 2 x2yz (fast) step 1: 2 x2y ⇌ x4y2 (fast) step 2: x4y2 + z2 → 2 x2yz (slow) step 1: z2 → z + z (slow) step 2: x2y + z → x2yz (fast) step 3: x2y + z → x2yz (fast) none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Consider the overall reaction: 2 x2y + z2 ⇌ 2 x2yz which has an experimentally determined rate law...
Questions




History, 30.11.2020 18:00

English, 30.11.2020 18:00



Mathematics, 30.11.2020 18:00

Social Studies, 30.11.2020 18:00



Mathematics, 30.11.2020 18:00

Biology, 30.11.2020 18:00




Mathematics, 30.11.2020 18:00



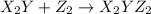 (slow)
(slow)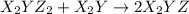 (fast)
(fast)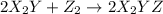
![Rate=k[X_2Y][Z_2]](/tpl/images/0304/3789/e6034.png)
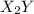 and
and 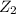 .
.![Rate=k[X_2Y]^2[Z_2]](/tpl/images/0304/3789/8a2d6.png)
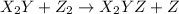 (fast)
(fast)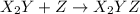 (slow)
(slow)![Rate=K'[X_2Y][Z]](/tpl/images/0304/3789/3c7d4.png) .............(1)
.............(1)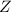 , we get:
, we get:![\frac{d[Z]}{dt}=K"[X_2Y][Z_2]](/tpl/images/0304/3789/87d2e.png) .........(2)
.........(2)![Rate=K'K"[X_2Y]^2[Z_2]](/tpl/images/0304/3789/e4aa1.png)
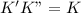
![Rate=K[X_2Y]^2[Z_2]](/tpl/images/0304/3789/5d2a6.png)
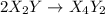 (fast)
(fast)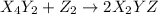 (slow)
(slow)![Rate=K'[X_4Y_2][Z_2]](/tpl/images/0304/3789/57b59.png) .............(1)
.............(1)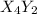 , we get:
, we get:![\frac{d[X_4Y_2]}{dt}=K"[X_2Y]^2](/tpl/images/0304/3789/bce7e.png) .........(2)
.........(2)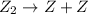 (slow)
(slow)![Rate=K[Z_2]](/tpl/images/0304/3789/c66c5.png)



