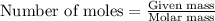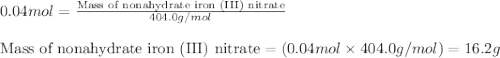In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess, some of the hydrate’s mass is water, and some is iron(iii) nitrate. how many grams of fe(no3)3•9h2o needed to be dissolved in water to make 2 l of 0.0020 m fe(no3)3? molecular weight of the nonahydrate is 404.0 g/mol. hint: try to set up an equation using x, and solving it. assume that the density of your solution is 1.000 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
In reality, a hydrate of iron(iii) nitrate had to be used, not the anhydrous salt. as you may guess,...
Questions



Biology, 28.01.2020 02:31



Mathematics, 28.01.2020 02:31









Social Studies, 28.01.2020 02:31

Advanced Placement (AP), 28.01.2020 02:31



Mathematics, 28.01.2020 02:31

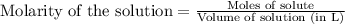
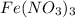 = 0.0020 M
= 0.0020 M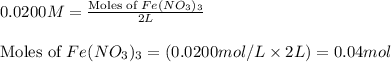
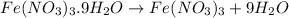
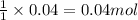 of hydrated iron (III) nitrate
of hydrated iron (III) nitrate