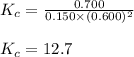At equilibrium, the concentrations of reactants and products can be predicted using the equilibrium constant, kc, which is a mathematical expression based on the chemical equation. for example, in the reaction aa+bb⇌cc+dd where a, b, c, and d are the stoichiometric coefficients, the equilibrium constant is kc=[c]c[d]d[a]a[b]b where [a], [b], [c], and [d] are the equilibrium concentrations. if the reaction is not at equilibrium, the quantity can still be calculated, but it is called the reaction quotient, qc, instead of the equilibrium constant, kc. qc=[c]tc[d]td[a]ta[b]tb where each concentration is measured at some arbitrary time t. part a a mixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.800 m , and [c] = 0.500 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.150 m and [c] = 0.700 m . calculate the value of the equilibrium constant, kc. express your answer numerically.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

You know the right answer?
At equilibrium, the concentrations of reactants and products can be predicted using the equilibrium...
Questions


Social Studies, 20.07.2019 04:50

Biology, 20.07.2019 04:50


History, 20.07.2019 04:50

Social Studies, 20.07.2019 04:50

History, 20.07.2019 04:50

Biology, 20.07.2019 04:50

History, 20.07.2019 04:50

Biology, 20.07.2019 04:50

Chemistry, 20.07.2019 04:50

Mathematics, 20.07.2019 04:50

Biology, 20.07.2019 04:50

Social Studies, 20.07.2019 04:50






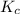
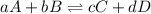
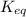 is written as:
is written as:![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0304/8312/9c8b0.png)
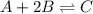
![[A]_{eq}=0.150M](/tpl/images/0304/8312/2394d.png)
![[C]_{eq}=0.700M](/tpl/images/0304/8312/9e4dd.png)
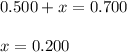
![[B]_{eq}=(0.800-x)=0.800-0.200=0.600M](/tpl/images/0304/8312/eb193.png)
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0304/8312/240ef.png)