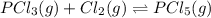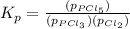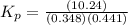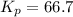Chemistry, 10.10.2019 00:10 GingerSnaps
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: pcl3 (g) + cl2 (g) → pcl5 (g) an equilibrium mixture at 450 k contains ppcl3 = 0.348 atm, pcl2 = 0.441 atm, and ppcl5 = 10.24 atm. what is the value of kp at this temperature? phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: (g) + (g) (g) an equilibrium mixture at 450 k contains = 0.348 atm, = 0.441 atm, and = 10.24 atm. what is the value of kp at this temperature? 66.7 1.50 ⋅ 10−2 12.99 1.57 9.45

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlor...
Questions

History, 16.11.2019 19:31


Mathematics, 16.11.2019 19:31


Mathematics, 16.11.2019 19:31




Mathematics, 16.11.2019 19:31

Mathematics, 16.11.2019 19:31

Mathematics, 16.11.2019 19:31


History, 16.11.2019 19:31

Social Studies, 16.11.2019 19:31



Mathematics, 16.11.2019 19:31

Business, 16.11.2019 19:31


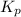 at this temperature is 66.7
at this temperature is 66.7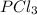 at equilibrium = 0.348 atm
at equilibrium = 0.348 atm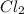 at equilibrium = 0.441 atm
at equilibrium = 0.441 atm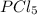 at equilibrium = 10.24 atm
at equilibrium = 10.24 atm