
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenolphthalein, is colorless in acidic solution but takes on a pink color in basic solution. how would the volume of standard solution added change if that solution were ba(oh)2(aq) instead of naoh(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenol...
Questions

Chemistry, 29.01.2020 14:45


Mathematics, 29.01.2020 14:45

Business, 29.01.2020 14:45

Biology, 29.01.2020 14:45


Health, 29.01.2020 14:45


Mathematics, 29.01.2020 14:45


Mathematics, 29.01.2020 14:45

Mathematics, 29.01.2020 14:45

Mathematics, 29.01.2020 14:45

English, 29.01.2020 14:45


Mathematics, 29.01.2020 14:45

English, 29.01.2020 14:45

Mathematics, 29.01.2020 14:45


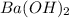 solution required will be half the volume required for NaOH solution.
solution required will be half the volume required for NaOH solution.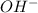 ion upon complete dissociation
ion upon complete dissociation ion. But one molecule of
ion. But one molecule of 


