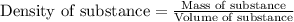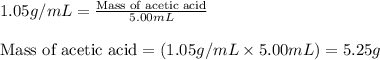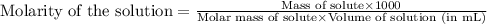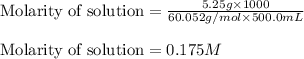"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml. pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml.
a. 3.50 × 10-5 m
b. 126 m
c. 0.0350 m
d. 2.10 m
e. 2.10 × 10-3 m

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity...
Questions

Mathematics, 26.09.2021 21:50


Mathematics, 26.09.2021 21:50


Physics, 26.09.2021 21:50

Biology, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50

Geography, 26.09.2021 21:50



French, 26.09.2021 21:50

Health, 26.09.2021 21:50

Health, 26.09.2021 21:50

English, 26.09.2021 21:50


English, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50