
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of pure magnesium metal. after sand papering the magnesium you place it in into your crucible and the mass of crucible, cover and magnesium masses out to be 24.66 grams. you observe that the magnesium is gray and shiny. you heat the crucible gently at first then vigorous for 10 minutes until the magnesium ignites. after the crucible cools you add a few drops of distilled water and heat again. the mass of the magnesium compound, crucible, and cover masses out to be 24.85 grams.
what is the empirical formula of the compound? show all work and units.
1) mass of the mg
2) mass of the mgo
3) mass of 0
4) moles of mg & moles of o
5) show mole to mole mgxoy
6) empricial formula of compound

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of p...
Questions



Computers and Technology, 11.03.2020 22:17


Mathematics, 11.03.2020 22:17

Computers and Technology, 11.03.2020 22:17



Mathematics, 11.03.2020 22:17











Geography, 11.03.2020 22:17

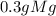 *
*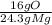 = 0.2g O
= 0.2g O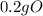 *
*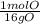 = 0.01mol O
= 0.01mol O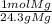 = 0.01mol Mg
= 0.01mol Mg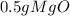 *
*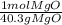 = 0.01mol MgO
= 0.01mol MgO

