
Chemistry, 10.10.2019 03:30 Imamdiallo18
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible mechanism for the reaction. group of answer choices
step 1: z2 --> z + z (slow) step 2: x2y + z → x2yz (fast) step 3: x2y + z → x2yz (fast) step 1: x2y + z2→ x2yz2 (slow) step 2: x2yz2 → x2yz + z (fast) step 1: 2 x2y + z2 → 2x2yz (slow) step 1: x2y + z2 → x2yz + z (slow) step 2: x2y + z → x2yz (fast) step 1: 2 x2y ⇌ x4y2 (fast) step 2: x4y2 + z2 → 2 x2yz (slow)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1


Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1
You know the right answer?
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible...
Questions

Mathematics, 03.11.2020 17:40





English, 03.11.2020 17:40

English, 03.11.2020 17:40





Mathematics, 03.11.2020 17:40






Chemistry, 03.11.2020 17:40

English, 03.11.2020 17:40

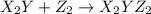 (slow)
(slow)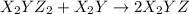 (fast)
(fast)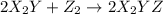
![Rate=k[X_2Y][Z_2]](/tpl/images/0305/6835/e6034.png)
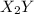 and
and 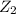 .
.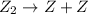 (slow)
(slow)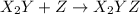 (fast)
(fast)![Rate=K[Z_2]](/tpl/images/0305/6835/c66c5.png)
![Rate=k[X_2Y]^2[Z_2]](/tpl/images/0305/6835/8a2d6.png)
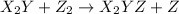 (fast)
(fast)![Rate=K'[X_2Y][Z]](/tpl/images/0305/6835/3c7d4.png) .............(1)
.............(1)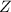 , we get:
, we get:![\frac{d[Z]}{dt}=K"[X_2Y][Z_2]](/tpl/images/0305/6835/87d2e.png) .........(2)
.........(2)![Rate=K'K"[X_2Y]^2[Z_2]](/tpl/images/0305/6835/e4aa1.png)
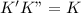
![Rate=K[X_2Y]^2[Z_2]](/tpl/images/0305/6835/5d2a6.png)
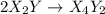 (fast)
(fast)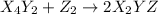 (slow)
(slow)![Rate=K'[X_4Y_2][Z_2]](/tpl/images/0305/6835/57b59.png) .............(1)
.............(1)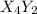 , we get:
, we get:![\frac{d[X_4Y_2]}{dt}=K"[X_2Y]^2](/tpl/images/0305/6835/bce7e.png) .........(2)
.........(2)


