
Chemistry, 10.10.2019 04:10 princess42044
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2. he pipets 20 ml of this solution into a 100 ml volumetric flask and dilutes to the mark. he then pipets 10 ml of this diluted solution into a 100 ml volumetric flask and dilutes to the mark. he analyzes some of the solution from the final volumetric flask and finds that the iodide ion concentration is 0.574 m. (note: in solution, calcium iodide breaks apart into one ca 2+ ion for every two i - ions, so a solution that is 1.0 m in cai 2 is 2.0 m in i determine the molar concentration of calcium iodide in the original solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2...
Questions


Biology, 14.10.2019 18:00



Mathematics, 14.10.2019 18:00


Mathematics, 14.10.2019 18:00



Biology, 14.10.2019 18:00

History, 14.10.2019 18:00

Chemistry, 14.10.2019 18:00

Social Studies, 14.10.2019 18:00

English, 14.10.2019 18:00

History, 14.10.2019 18:00


Physics, 14.10.2019 18:00

Physics, 14.10.2019 18:00


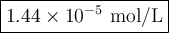
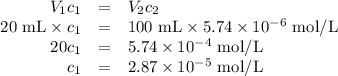
![[\text{Ca}^{2+}] =\dfrac{2.87 \times 10^{-5} \text{ mol I}^{-}}{\text{1 L}} \times \dfrac{\text{1 mol Ca}^{2+} }{\text{2 mol I}^{-}} = \mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}\\\\\text{[Ca$^{2+}$] in the original solution was $\large \boxed{\mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}}$}](/tpl/images/0305/8108/dfb7a.png)


