
Chemistry, 10.10.2019 04:10 emmabarnett817
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. view available hint(s) what is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00 l of 0.0100 m benzoic acid, hc7h5o2? assume that there is no change in volume. the ka for benzoic acid is 6.3 × 10–5. 4.33 3.76 0.439 4.64

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
What is the ph of a buffer that was prepared by adding 3.96 g of sodium benzoate, nac7h5o2, to 1.00...
Questions

Health, 19.11.2020 17:40

English, 19.11.2020 17:40


English, 19.11.2020 17:40





Mathematics, 19.11.2020 17:40


Mathematics, 19.11.2020 17:40


Mathematics, 19.11.2020 17:40






Mathematics, 19.11.2020 17:40

Mathematics, 19.11.2020 17:40

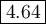
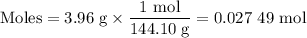
![[\text{A}^{-}] = \dfrac{\text{0.027 49 mol}}{\text{1 L}} = \text{0.027 49 mol/L }](/tpl/images/0305/8117/b8b4c.png)
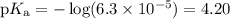
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log\dfrac{[\text{A}^{-}]}{\text{[HA]}}\\\\& = & 4.20 +\log\dfrac{0.02749}{0.0100}\\\\& = & 4.20 + \log2.749 \\& = & 4.20 + 0.4390\\& = & 4.64\\\end{array}\\\text{The pH of the buffer is $\large \boxed{\mathbf{4.64}}$}](/tpl/images/0305/8117/4e2e9.png)


