
Chemistry, 10.10.2019 05:00 akaeiraspruell
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to carbonate (co32-) to determine: hco3 = co2 + h+ k = 10-10.33 (a) whether hco3 or co32- would dominate at ph 9.1 and (b) what the concentration of [co32-] would be at this ph if [hco3 ] = 10-6 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to...
Questions




History, 25.10.2019 00:43

Computers and Technology, 25.10.2019 00:43





English, 25.10.2019 00:43





English, 25.10.2019 00:43

Mathematics, 25.10.2019 00:43


Physics, 25.10.2019 00:43


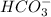 will dominate at pH = 9.1
will dominate at pH = 9.1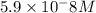
![pH=-\log[H^+]](/tpl/images/0305/9437/cf945.png) ......(1)
......(1)![9.1=-\log[H^+]](/tpl/images/0305/9437/e5ddb.png)
![[H^+]=10^{-9.1}](/tpl/images/0305/9437/b7f9d.png)
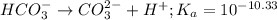
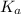 for above reaction follows:
for above reaction follows:![K_a=\frac{[CO_3^{2-}]\times [H^+]}{[HCO_3^-]}](/tpl/images/0305/9437/fea1b.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{[HCO_3^-]}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=\frac{10^{-9.1}}{10^{-10.33}}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=16.98](/tpl/images/0305/9437/786f2.png)
![[HCO_3^-]=16.98\times [CO_3^{2-}]](/tpl/images/0305/9437/e6ce5.png)
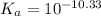
![[HCO_3^-]=10^{-6}M](/tpl/images/0305/9437/1335d.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{10^{-6}}](/tpl/images/0305/9437/27401.png)
![[CO_3^{2-}]=\frac{10^{-6}\times 10^{-10.33}}{10^{-9.1}}=5.9\times 10^-8}M](/tpl/images/0305/9437/65347.png)


